Geothermal Lithium: The Final Frontier of Decarbonization
Robert Pell, President, Minviro, London, UK
Alex Grant, Principal, Jade Cove Partners, San Francisco, USA
David Deak, President, Marbex, Palo Alto, USA
May 2020
The PDF of this article is available here and its associated LinkedIn post is available here.
In the early 2020s, we find ourselves in the middle of a transition in how we extract, process, transport, and use energy. In the last century, we have cut the time it takes to travel from Los Angeles to Tokyo from days to hours. We have developed ways to communicate instantly with anyone on the planet by video chat. We have built supply chains that make bananas available in Finland in the middle of winter. All of this was made possible by the extraction, processing, transport, and ultimately combustion of energy dense fossil fuels.
We now understand that this final step in the energy system, reaction of a high energy carbon chain with oxygen in air, is emitting enough CO2 into the atmosphere that it will soon make our climate much less stable than we are used to. There is no guarantee that we’ll be able to maintain our quality of life if the climate spirals out of control.
In this century, we will change the way we extract, process, transport, and use energy away from fossil fuel foundations. We will extract energy and convert it into electric potential on electrons. The amount of energy we use to move those electrons will be a tiny fraction of the total energy we capture, so most of the energy we capture will make its way to its end use. Today, in the United States we use ~67% of the energy we extract from nature to process it, transport it, and waste it instead of using it in useful energy services. (1) Reducing the amount of energy we waste (shown as “rejected energy” below) goes hand in hand with transitioning the way we store and transport energy from carbon molecules to electrons.
One attractive feature of using fossil fuels is that energy is delivered, stored, and handled in small volumes. Today, there is growing consensus that the best way to store the energy of electrons for mobility applications is in lithium-ion batteries (LIBs). LIBs do not directly emit CO2 and they do not require chemical reactions or heat to function. Lithium is a good choice for batteries because we can store an electron of electric potential on the lithium atom, while its location in the top left of the Periodic Table means that it carries along the fewest neutrons and protons. This means it carries more energy per unit mass or volume. LIBs can also be charged and discharged with high efficiency, so typically >99% of the energy put in comes back out. This is why LIBs are now used in electric vehicles.
From now until 2030, most industry analysts expect the net consumption of lithium for LIB manufacturing to increase by a factor of 8-10x from 2020. (2) This has sparked a major soul-searching question for the battery industry. Where will the lithium come from, and are the ways we have produced lithium in the past ideal for the ways we will need to produce it in the future?
Until the 2020s, we only extracted lithium from two types of natural resources: spodumene (hard rock) and brines from high concentration, low impurity basins in South America. The best-known deposits of these resources are already developed into operating assets. Further, evaporative brine operations in South America are sensitive to weather/climate variations, political issues in Chile & Argentina, and complex hydrogeology which have slowed their development. Most notably though, lithium buyers are concerned that brine extraction may impact freshwater resources for people and habitats near those operations. Some purchasers have even stopped buying lithium chemicals from brine projects because of this.
This is one reason why most growth in lithium production in the 2010s has been captured by spodumene mining in Australia, with downstream conversion in China. It is dependable and relatively simple to extract and concentrate. Further, it lends itself well to making LiOH٠H2O, which brines conventionally have only been able to produce by first producing Li2CO3. The major drawback of spodumene processing is that it requires a large quantity of thermal energy to modify the spodumene crystal before the lithium can be extracted and refined with acid. In China, this is done by burning coal, and this is also where the majority of spodumene conversion takes place. Many in the industry intuited that this must result in a major CO2 footprint. Lithium buyers are now wondering if there may be other ways to produce lithium apart from spodumene and South American brines.
A compelling class of projects is in development which seek to extract lithium from deep, hot geothermal brines in places like California and Germany, producing lithium chemicals and low CO2 intense electricity simultaneously. These projects plan to use direct lithium extraction (DLE) to remove the lithium from the brine after energy has been extracted, then to pump all of the water and other salts in the brine back underground. Some of them propose to use little or no fossil fuels to power their operations.
Geothermal lithium projects could kill two birds with one stone: producing low CO2 intensity lithium chemicals for LIB manufacturing and decarbonizing electricity grids simultaneously. This is distinctly different from how lithium is produced using evaporative processes in South America for two reasons.
First, in evaporative processes, ~98% of the water in the brine is evaporated using solar energy in evaporation ponds covering tens of square kilometers. In geothermal lithium projects, DLE could be used to extract lithium selectively from a brine without evaporating the water from the brine, while an energy plant is used to extract the heat from the brine to produce power. Second, in evaporative processes, the brine comes from shallow aquifers (~10m deep) and the energy used to evaporate the water from the brine comes from the sun. In geothermal lithium brines, the lithium and energy come from deep aquifers (~2km deep) co-packaged in the same brine.
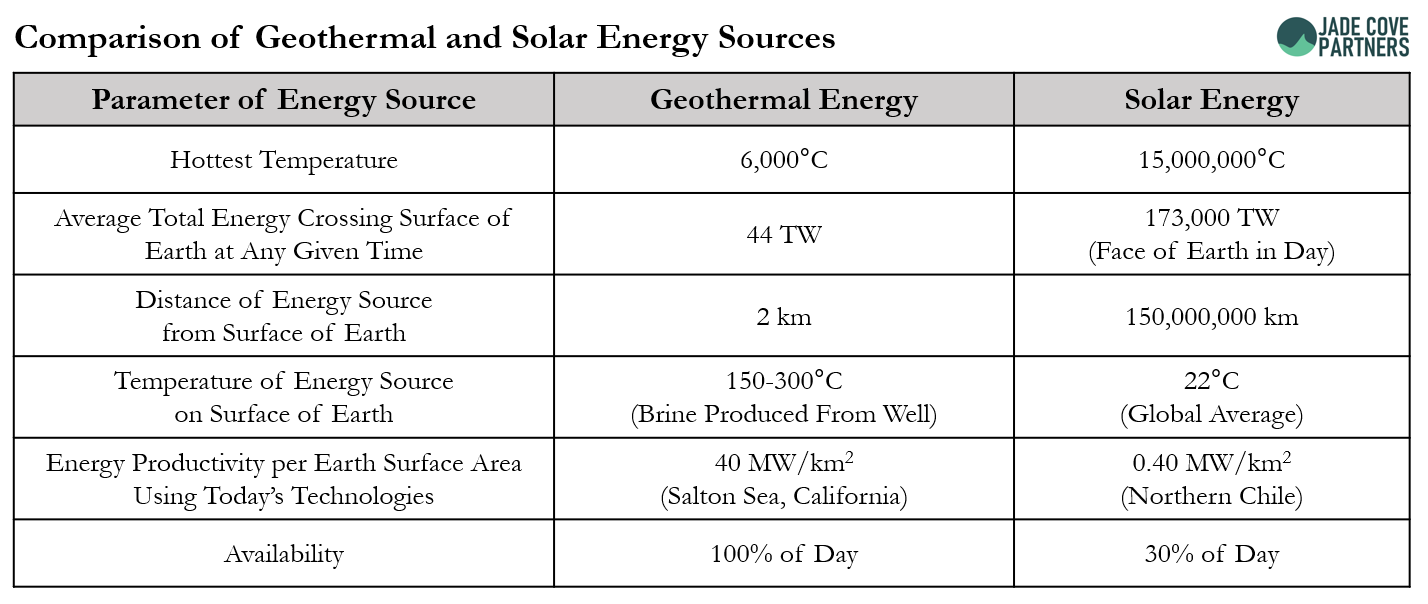
Geothermal energy is ~160x more energy dense on the surface of the earth than solar energy is and is available 24/7, while the sun is only available a third of the time. This means that 1km2 of the Earth’s surface, where the geothermal gradient is suitable for energy extraction, can produce much more energy from geothermal brines than it can from solar insolation. Geothermal energy shares one of the key attributes of fossil fuels that our new energy system could benefit from: high energy density. In the lithium context, the difference between geothermal lithium and evaporative process-produced lithium chemicals is even more stark because of the efficiency of energy consumption in the processes. Obviating the need to evaporate the water from the brine, geothermal lithium products may net consume ~100x less energy than evaporative processes. Meanwhile, exporting low CO2 power to the grid while evaporative processes do not produce any power as a co-product. Combining the energy intensity of the different energy sources on the Earth’s surface and the differences in water evaporation requirements for evaporation ponds and DLE projects, we find that geothermal lithium projects could require ~10,000x less physical footprint than evaporative projects to produce the same amount of lithium chemical.
Below, we have plotted lithium concentration in different brine resources vs. the total energy consumption to produce the lithium chemical, and the physical footprint of extracting the lithium from those brines. Two geothermal lithium projects are included, and the one evaporative lithium process represented is a Chilean operation. Though the solar energy used to evaporate the water has a low CO2 intensity, the vast majority of it is used to evaporate water, which is not necessary if using DLE. Could that energy be captured by solar photovoltaic instead, and only a small fraction of it used to extract the lithium instead? If the social license to operate evaporative brine processes is lost, and brine project expansion plans are forced to use DLE, then maybe it could unlock new opportunities for solar energy production and electric grid decarbonization?
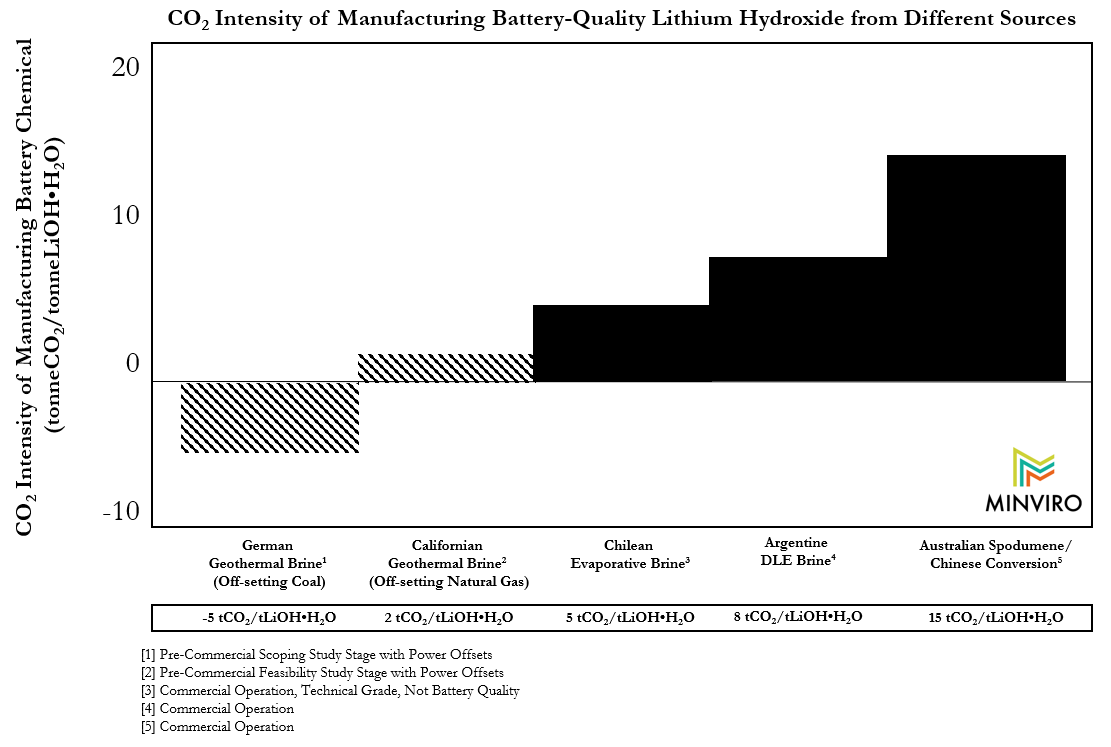
In January 2020, we published the first ever life cycle assessment (LCA) of different processes to produce LiOH٠H2O for modern cathode manufacturing. Our study quantified the CO2 emissions of spodumene mining in Australia and conversion in China, and found that it was 6-7x more CO2 intense than producing lithium from brine resources using evaporative processes. (3)
Considering that 8-10x more lithium extraction and chemical production is required to manufacture batteries by 2030, and we are transitioning our energy system to avoid these emissions, lithium buyers should strongly consider favoring lithium chemicals which emit as little CO2 as possible in production. It is important to quantify this CO2 emission mitigation opportunity. In the same LCA, we contemplated two geothermal lithium projects in development. Below are results for those two projects published for the first time: one in Germany, and one in California.
The geothermal lithium projects contemplated in the LCA are in different stages of planning, and are both envisioned to be constructed within the next 10 years. They have different impacts for a couple reasons.
In LCA models, it is common to use “off-sets”, or a negative CO2 intensity component of the operation’s emissions, if a co-product displaces high CO2 intensity activity elsewhere. In this case, that co-product is power. In California, the grid is relatively clean while in Germany the grid’s baseload power is coal. Though theoretically the two operations could have similar gross CO2 emissions, their off-sets are different, thus their net CO2 emissions would be different because they are decarbonizing different grids.
Further, the way geothermal energy is produced in Germany typically allows for less CO2 emissions, which can come out of the brine if the pressure is dropped. In both cases, it is possible to use steam and electricity from the geothermal plant to drive the lithium processing with no fossil fuel inputs. This means that one dense form of energy that emits CO2 is displaced for another dense form of energy that operationally emits none. This could only be done in the solar context if the entire lithium processing flowsheet was electrified, but geothermal brines can also directly produce heat. This is a unique feature of the source of energy for the process.
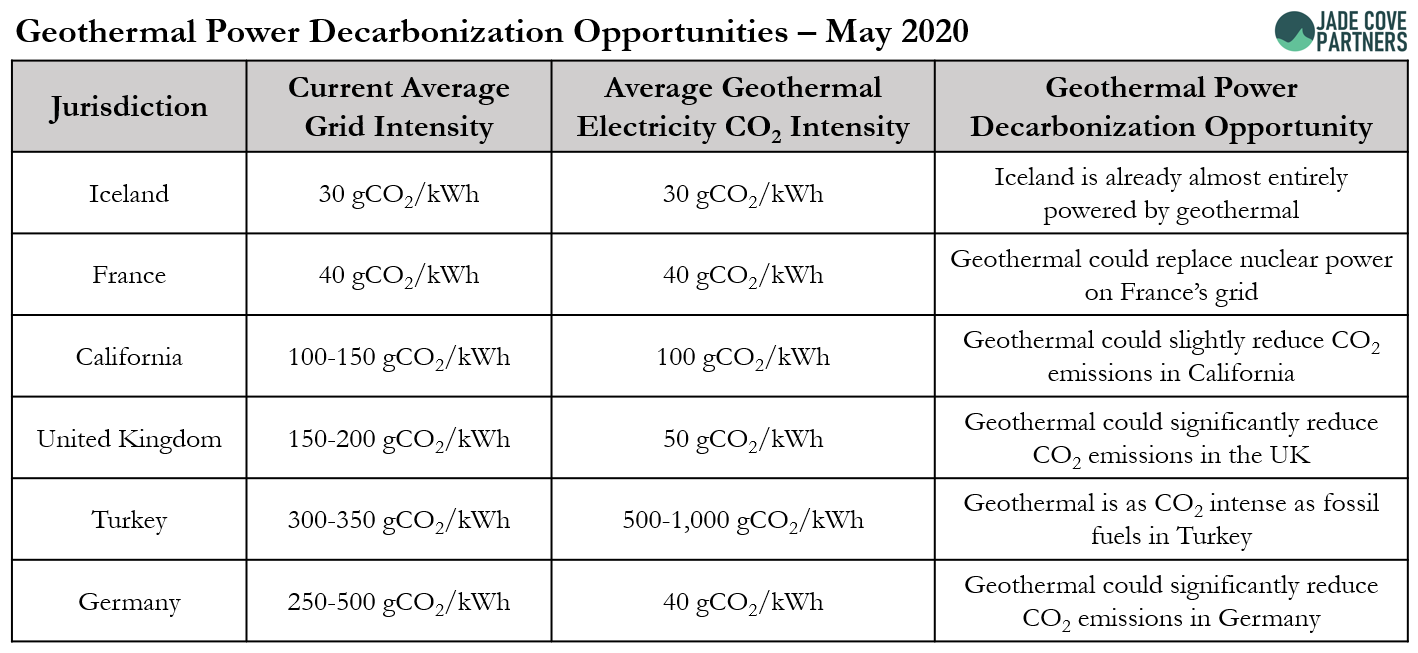
Below is a table of emissions for geothermal energy producing grids around the world, which hints at why there are differences of low CO2 intensity power off-sets between different geothermal projects. (4)
Geothermal lithium projects are an exciting opportunity to accelerate the decarbonization of transportation. Geothermal lithium could be a way to reduce both the embodied emissions of manufacturing electric vehicles, and the emissions associated with charging EVs simultaneously. This is the final frontier of decarbonization, and this is why we believe that lithium buyers, governments, and other agencies should commit to helping these exciting projects get built. We welcome the California Energy Commission’s funding of geothermal lithium initiatives, (5) and hope that it results in material commercial activity. More capital should be invested into geothermal lithium brine exploration as well, since there may be other geothermal brinefields around the world that could facilitate these kinds of projects, and realize the same kinds of decarbonization opportunities.
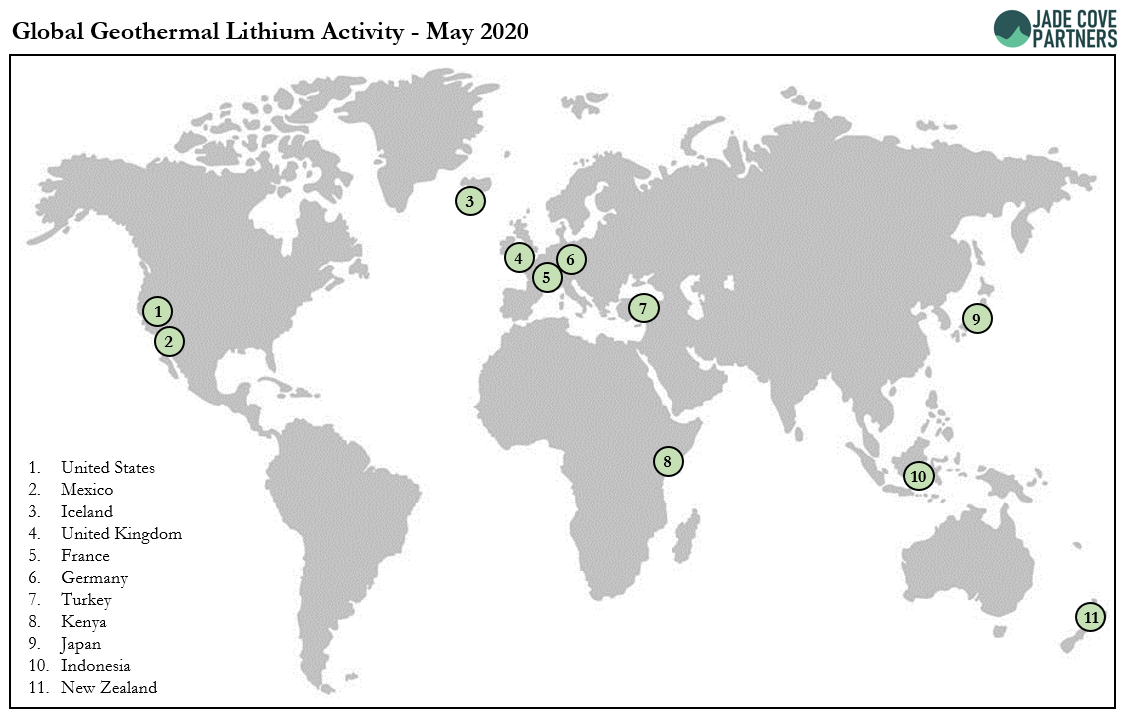
We hope that multiple projects like this can be built in the 2020s, and that they will help accelerate the world’s transition to renewable energy. The Salton Sea in California and the Upper Rhine Valley of France & Germany are the two most well-known geothermal lithium brinefields, and will represent test-beds for the concept.
Below is a global overview of geothermal lithium activity to date.
Acknowledgements
We’d like to thank EnergySource Minerals LLC and Vulcan Energy Resources Ltd. for co-sponsoring this study. Both sponsoring companies had no influence on the process or results of the LCA analysis. Thank you to Pedro Mauricio Torres of Beyond Lithium Consultores for helpful information on South American brine operations. Thank you also to Matt Uddenberg of Stravan Consulting for insight into enthalpies of geothermal brines.
References
(1) Lawrence Livermore National Lab, 2019. Estimated U.S. Energy Consumption in 2019. URL.
(2) Benchmark Mineral Intelligence, 2020. Lithium Demand Forecast. URL.
(3) Alex Grant, Robert Pell, and David Deak, 2020. The CO2 Impact of the 2020s Battery Quality Lithium Hydroxide Supply Chain. URL.
(4) ElectricityMap. Live CO2 Emissions. URL.
(5) ThinkGeoEnergy. California Energy Commission awards $10m in grants to geothermal, lithium recovery projects. URL.