The Sedimentary Lithium Opportunity
Alex Grant, Principal, Jade Cove Partners, San Francisco, USA
October 2019
The PDF of this article is available here and its associated LinkedIn post is available here.
Lithium is an essential component of lithium-ion batteries which occurs abundantly in the Earth’s crust in many different forms, roughly classified as pegmatites (“hard rock”), brines, and sedimentary deposits (which is sometimes erroneously generalized as “clay”). Lithium does not sort itself into tidy buckets, but its forms in nature can be roughly categorized in these three families.
Currently, only pegmatite and brine resources are used to produce lithium chemical products commercially at large scale, but a host of new players aiming to produce lithium from sedimentary resources in Western North America and around the world are emerging. The sedimentary resource projects claim to take advantage of favorable chemistry of processing the sediments, sometimes described as the “best of both worlds”, when compared to pegmatites and brines.
In this article, I will share some of the most promising features of sedimentary resource projects, who’s working on developing these deposits, and why capital markets should take them seriously as future sources of lithium chemicals. It will be helpful to understand some of the pros and cons of processing pegmatites and brines into lithium chemicals to understand the “best of both worlds” argument for the sedimentary deposits.
Pegmatite
In pegmatites, lithium is strongly bound in crystal structures like aluminosilicates (Al, Si oxides) and because the lithium is so tightly bound, the mineral requires aggressive processing to remove it to make lithium chemicals. Spodumene is the most widely mined lithium-bearing pegmatite, and has been successfully commercially developed into a significant source of lithium chemicals (representing around half of global supply in 2019). It is first mined and crushed to smaller pieces. The crushed material is then upgraded to remove waste materials from the deposit that are not spodumene and don’t contain lithium. Once upgraded, calcination (heating to ~1,000°C) is used to convert the crystal to a different crystal phase that is more amenable to extracting the lithium. These high temperatures are typically generated using fossil fuels, meaning the carbon footprint of calcining pegmatites is typically higher than processing of other lithium resources. Calcination is a fundamental aspect of extraction of lithium from spodumene because of its crystal structure, and it is difficult to get around this. Some other pegmatites may not require this roasting step.
This calcination process is followed by a chemical treatment to extract the lithium. This gives a mostly pure lithium concentrate (called the leachate) which can be refined into lithium chemicals with a relatively simple technological approach involving addition of chemicals and temperature changes. Pegmatites are a good source of lithium because they are easy to manipulate from a mining engineering perspective, and the leachate obtained isn’t heavily contaminated with elements with similar chemical characteristics to lithium (ex. alkali/alkaline earths like Na, K, Mg, Ca, Sr), meaning the impurities are easy to remove from the leachate. The waste produced from spodumene operations can be stacked or used for other applications like concrete manufacturing. Lithium can be produced from other minerals like lepidolite and zinnwaldite using similar flowsheets, but some modifications are required depending on the unique mineralogy.
Brine
Brine resources are very different from pegmatites from a lithium extraction and processing perspective. Brines are high concentration salty waters in which salts are dissolved (ex. Li, Na, K, Mg, Ca, Sr are common cations, or positively charged species, while Cl, SO4, BO3, and CO3 are common anions, or negatively charged species). The minerals in brines start off as volcanic materials but over millions of years, rain and geochemical phenomena cause them to dissolve in water and concentrate in basins. Brines can be as high as 20-40% salt by mass, meaning that if you were to evaporate away the water from the brine, around 20-40% of the mass would be left behind as salt crystals. Brines are liquid, meaning that they need to be pumped to the surface for processing, not dug up and crushed like pegmatites. This means that they do not require roasting or leaching operations to put the lithium into solution for further processing: the lithium is already dissolved. There are two ways to remove lithium from brines.
First, evaporative processes can be used to evaporate the water from the brine, leaving behind contaminant salts and a concentrate of (ideally) mostly lithium chloride which can be processed into lithium chemicals. This process only works economically for high lithium concentration brines with low impurities in places with minimal rainfall. Further, there is concern that if brine is pumped out, freshwater aquifers sitting on top of brine aquifers may be impacted, causing water availability issues.
Second, direct lithium extraction (DLE) processes can be used to remove lithium from the brine to produce a concentrate, leaving behind a “spent brine” containing all the original components of the natural brine but without the lithium. This spent brine needs to be re-injected and/or separated from the fresh brine so the two don’t mix, or else the natural lithium-bearing brine will be diluted by the spent brine, making it impossible to extract more lithium.
Sedimentary
Sedimentary deposits are considered to share some of the positive attributes of both pegmatites and brines. Sedimentary resources are created when lithium is washed out of volcanic minerals into basins where it reacts with other minerals, creating chemical structures in which the lithium is bound up in a mineral, but much less strongly bound compared to spodumene. Sediments typically have the consistency of dirt, not hard rock, and sometimes easily break up when placed in water. If the lithium was not bound in a mineral at all, it would wash out in water forming a brine (this is typically not observed). A number of leading projects are proposing not using calcination in their sediment processing flowsheets, meaning the lithium is bound in the mineral with a lesser strength compared to pegmatites. A chemical leach is used to extract the lithium from the sediment, after which the waste sediment can be stacked or back-filled into an open pit.
The lack of requirement to roast the sediment is a positive attribute for these resources because it means that fossil fuels may not be needed to process the sediments. Some projects report requiring upgrading of the sediment ore to remove contaminants which would unnecessarily consume acid, and in October 2019, only one project is proposing to use a calcination in their flowsheet. The benefit of processing a sediment containing “loosely bound” lithium is that the solid waste can be easily disposed of without diluting the original resource, similar to the waste materials from pegmatite processing.
The sedimentary resource projects have some promising attributes for a future of supplying lithium to the battery industry, but reagent inputs will need to be optimized thoroughly for each individual project. Every sediment is different, and the flowsheets of the different projects may look quite different. The chemistry of the sediments varies significantly (which is also the case for brines), and each project will need to take this into account. Currently, most public pre-feasibility studies show that tens to hundreds of times excess of reagents are used to create the lithium leachates. This implies low lithium concentrations in the leachate compared to pegmatite-derived leachates, and high concentrations of impurities like Na, K, and Mg. This explains why most projects currently propose by-product sales to reduce apparent OPEX (electricity, sulfuric acid, boric acid, potash, etc.) because these are likely high OPEX flowsheets if they were “pure play” lithium. Further, the high porosity and low particle size of the sediments mean that they “hold on” to leachate during leaching, and optimizing solid/liquid separations will be key to extracting most of the lithium from the spent ore. When this is done poorly, the ore may “gum up” and a significant amount of lithium can be lost with the waste.
The “in between” strength of how lithium is chemically bound in sediments results in some of their “best of both world” characteristics when compared to brines and pegmatites, and these strengths should be taken advantage of in future flowsheet development. New leaching techniques and reagent management flowsheets may be helpful in unlocking these sedimentary materials to produce high lithium concentration, low impurity concentration leachates that can be more easily processed into battery-quality lithium chemicals. The sedimentary deposit lithium projects are young, but I believe that some of them will be built in the 2020s. The healthy mining jurisdiction of Western North America, proximity of the deposits to American battery manufacturers, and possibility for low carbon intensity means that they have excellent potential for supplying lithium for batteries in the near future, and that they should be followed closely.
A summary of the typical process pathways for lithium chemical manufacturing from different types of resources is shown below.
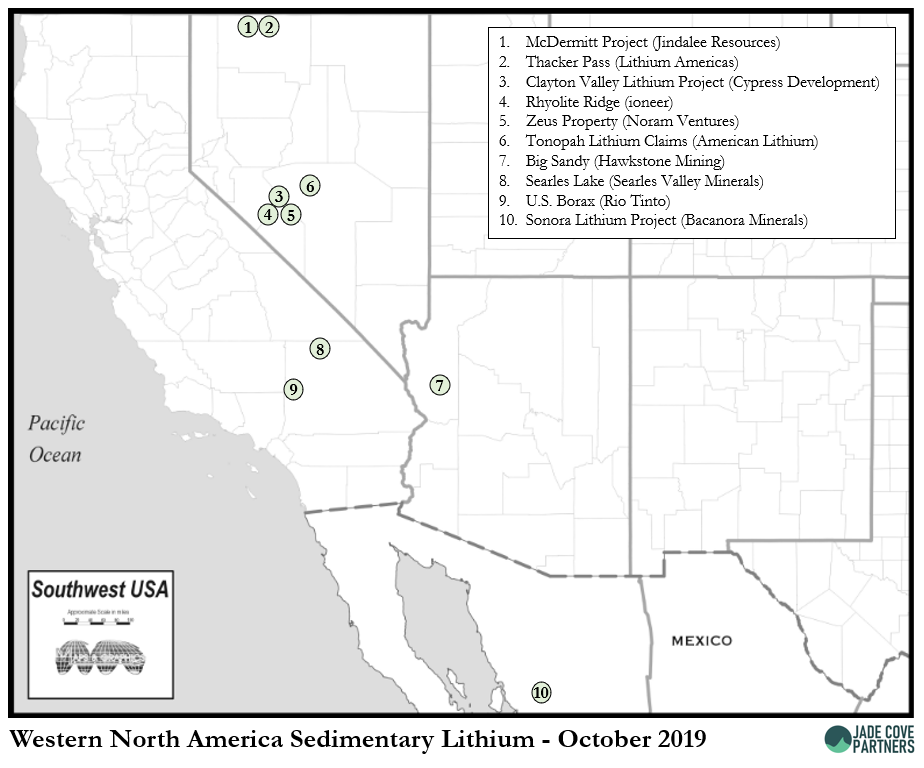
A map of these projects in Western North America with public information in October 2019 is below.
Acknowledgments
Thank you to those who influenced the composition of this article including Anna Wall, Tom Benson, and David Deak.